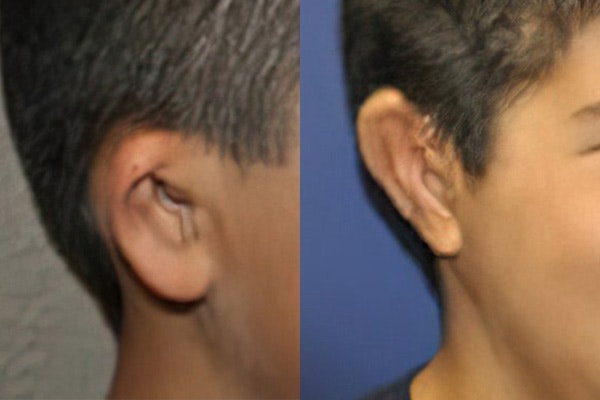
The Future of Microtia Surgery
 .
.
3DBio Therapeutics, Long Island City, NY
Study Title
A Multicenter, Single Arm, Prospective, Open-Label, Staged Study of the Safety and Efficacy of the AuriNovo Construct for Auricular Reconstruction in Subjects with Unilateral Microtia
Investigational Product
The investigational product is called AuriNovo™ and it is not currently approved by the U.S. Food and Drug Administration (FDA). AuriNovo is a patient-specific, biologic implant for use in surgical reconstruction of the external ear (auricle) in people born with microtia Grades II-IV. It is 3D-bioprinted using the patient’s own auricular cells combined with collagen. It includes a biodegradable overshell made of polydioxanone. The study is being conducted under the FDA’s Investigational New Drug/Biologic (IND) program.
Purpose of the 3D Cartilage Printing Study
This is a first-in-human (Phase 1/2a) study being conducted to collect preliminary safety and efficacy data on AuriNovo. Data also are being collected on technical, logistical, surgical, and post-surgical care aspects related to implantation of AuriNovo.
Protocol Summary
The surgeon (Principal Investigator) will work with 3DBio manufacturing to design an AuriNovo implant that is sized and shaped specifically for a given patient. At the time of the microtia reconstruction surgery, the surgeon will place the overshell component parts around the biologic components, suture the outside overshell parts together, and implant the entire AuriNovo product (overshell and biologic components) under the skin or skin flap. The implantation procedure varies based on the surgeon’s chosen technique.
The study subject, subject’s caretaker (if the subject is not an adult), and the surgeon will complete questionnaires indicating their level of satisfaction with the AuriNovo implant. Safety data will be collected at each visit.
Enrollment for the entire study is limited to 11 subjects aged 6-25 years old. Since the study is small, enrollment is largely by invitation from the surgeon (Principal Investigator).
It is anticipated that it will take approximately 18 to-21 months to recruit the targeted number of subjects due to staged (spread out over time) enrollment and up to 5 additional years to complete the long-term follow up.
Basic Study Eligibility Criteria
(Below is a partial list of study eligibility criteria)
- 6-25 years old, inclusive
- Born with unilateral microtia Grade II, III, or IV
- No previous surgical procedure for auricular reconstruction
- Have undergone an audiological assessment prior to enrollment and surgeon confirmation that study participation will not preclude future hearing correction options
- Normal or near normal hairline position
- Healthy subjects with no history of cancer, problematic wound healing, or immune disorders
Patients who meet any of the following exclusion criteria are not eligible for the study:
- Previous cochlear implant surgery
- Prior surgery in the temporal, parietal, or mastoid regions of the affected side that resulted in scarring that may affect the outcome of microtia reconstruction surgery.
- Sensitivity to broad-spectrum aminoglycoside antibiotics containing any of the following: streptomycin, gentamycin tobramycin, amikacin, kanamycin, neomycin, or plazomicin.
- Sensitivity to materials of porcine origin including pork products. For subjects with no known exposure to porcine materials including pork products, sensitivity as confirmed at baseline through a positive porcine skin sensitivity test.
- Patients previously diagnosed/evaluated for any of the following syndromes:
- CHARGE (Coloboma, Heart defect, choanal Atresia, Retarded growth and development, Genital hypoplasia, Ear anomalies) syndrome,
- Branchio-oto-renal (BOR) syndrome
- Patients with renal dysfunction of any etiology
Further information on this study is available on clinicaltrials.gov.